Probing the mechanism for graphene nanoribbon formation on gold surfaces through X-ray spectroscopy
Abstract
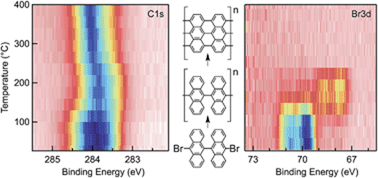
We studied the formation of graphene nanoribbons (GNRs) via the self-assembly of 10,10′-dibromo-9,9′-bianthryl precursor molecules on gold surfaces with different synchrotron spectroscopies. Through X-ray photoemission spectroscopy core-level shifts, we followed each step of the synthetic process, and could show that the Br–C bonds of the precursors cleave at temperatures as low as 100 °C on both (111) and Au(110). We established that the resulting radicals bind to Au, forming Au–C and Au–Br bonds. We show that the polymerization of the precursors follows Br desorption from Au, suggesting that the presence of halogens is the limiting factor in this step. Finally, with angle-resolved ultraviolet photoemission spectroscopy and density functional theory we show that the GNR/Au interaction results in an upshift of the Shockley surface state of Au(111) by ∼0.14 eV, together with an increased electron effective mass.