Abstract
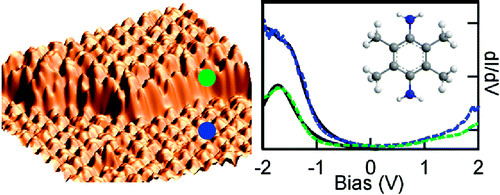
We investigate the binding and energy level alignment of 2,3,5,6-tetramethyl-1,4-benzenediamine (TMBDA) on Au(111) through a combination of helium atom scattering (HAS), X-ray photoemission (XPS), and scanning tunneling microscopy (STM). We show that TMBDA binds to step edges and to flat Au (111) terraces in a nearly flat-lying configuration. Through combination of HAS and STM data, we determine that the molecules are bound on step edges with an adsorption energy of about 1.2 eV, which is about 0.2 eV stronger than the adsorption energy we measure on flat surface. Preferential bonding to the under-coordinated Au atoms on step edges suggests that the molecules bind to Au through the nitrogen lone pair. Finally, STM measurements on TMBDA in these two different adsorption configurations show that the highest-occupied molecular orbital is deeper relative to Fermi for the more strongly bound molecules on step edges, confirming that the nitrogen bonds through charge donation to the Au.