Intermolecular Hydrogen Bonding and Molecular Orbital Distortion in 4-Hydroxycyanobenzene Investigated by X-ray Spectroscopy
Abstract
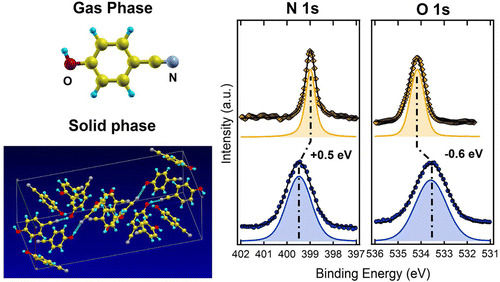
Electronic structure of 4-hydroxycyanobenzene in the gas phase, thick films, and single crystals has been investigated by X-ray photoemission spectroscopy (XPS) and near edge X-ray absorption fine structure spectroscopy (NEXAFS). We have used resonant photoemission spectroscopy (RESPES) to identify the symmetry and atomic localization of the occupied and unoccupied molecular orbitals for the free molecule. Upon condensation into a thick film, we find XPS energy shifts in opposite directions for the oxygen and nitrogen core levels, consistent with the formation of an intermolecular hydrogen bond. This interaction is also accompanied by a significant spatial distortion of the lowest unoccupied molecular orbital that is displaced from the nitrogen atom, as indicated by the RESPES measurements. Thick films and single crystals display the same dichroism in polarization dependent NEXAFS, indicating that the intermolecular hydrogen bonding also steers the molecular assembly into a preferred molecular orientation.